Chemical Equilibrium - Video Lecture
More Chemistry Free Demo Videos
![]() Introduction
0:00
Introduction
0:00
![]() Equilibrium
0:50
Equilibrium
0:50
![]() Dynamic Equilibrium
3:39
Dynamic Equilibrium
3:39
![]() Dynamic Physical Equilibrium
9:16
Dynamic Physical Equilibrium
9:16
![]() Solid Liquid Equilibrium
10:16
Solid Liquid Equilibrium
10:16
![]() Liquid-Vapour Equilibrium
12:01
Liquid-Vapour Equilibrium
12:01
![]() Solid-Vapour Equilibrium
12:27
Solid-Vapour Equilibrium
12:27
![]() Dissolution of Solid in Liquids
14:43
Dissolution of Solid in Liquids
14:43
![]() Dissolution of Gases in Liquids
16:38
Dissolution of Gases in Liquids
16:38
![]() Characteristics of Physical Equilibrium
19:25
Characteristics of Physical Equilibrium
19:25
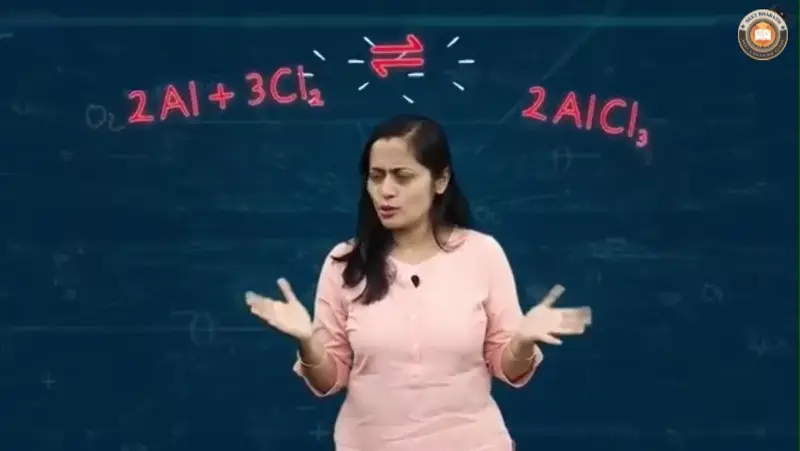
![]() Chemical Equilibrium
20:02
Chemical Equilibrium
20:02
![]() Chemical Equilibrium Example
25:33
Chemical Equilibrium Example
25:33
![]() Reversible reactions:Examples
26:33
Reversible reactions:Examples
26:33
![]() Depiction of Equilibrium
27:33
Depiction of Equilibrium
27:33
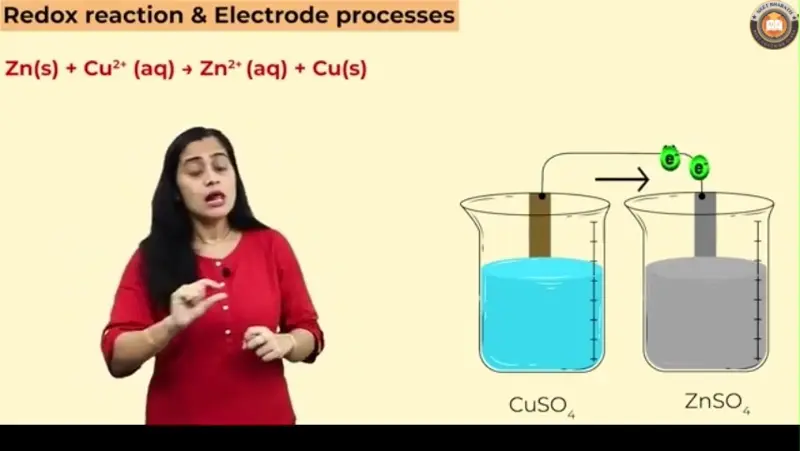
![]() Chemical Equilibrium:Characteristics
29:35
Chemical Equilibrium:Characteristics
29:35
![]() Law of Mass Action
31:44
Law of Mass Action
31:44
![]() Equilibrium Constant
33:03
Equilibrium Constant
33:03
![]() Law of chemical equilibrium
36:02
Law of chemical equilibrium
36:02
![]() Steps for writing equilibrium constant
39:08
Steps for writing equilibrium constant
39:08
![]() Equilibrium constant in Gaseous System
43:29
Equilibrium constant in Gaseous System
43:29
![]() Hetergeneous 7 Homogeneous Mixture
51:44
Hetergeneous 7 Homogeneous Mixture
51:44
![]() Characteristics of Equilibrium constant
52:40
Characteristics of Equilibrium constant
52:40
![]() Applications of Equilibrium constant
1:02:13
Applications of Equilibrium constant
1:02:13
![]() Predict the direction of the reaction
1:05:10
Predict the direction of the reaction
1:05:10
![]() Example on predicting the direction of the reaction
1:07:27
Example on predicting the direction of the reaction
1:07:27
![]() Example 1 on calculating equilibrium constant
1:10:31
Example 1 on calculating equilibrium constant
1:10:31
![]() Example 2 on calculating equilibrium constant
1:17:10
Example 2 on calculating equilibrium constant
1:17:10
![]() K,Q And G relationship
1:21:43
K,Q And G relationship
1:21:43
![]() Example : K,Q And G relationship
1:26:38
Example : K,Q And G relationship
1:26:38
![]() Factors affecting equilibrium
1:27:32
Factors affecting equilibrium
1:27:32
![]() Le Chatelier's Principle
1:30:07
Le Chatelier's Principle
1:30:07
![]() Effect of concentration change
1:31:43
Effect of concentration change
1:31:43
![]() Effect of change of pressure
1:34:24
Effect of change of pressure
1:34:24
![]() Effect of change of volume
1:38:00
Effect of change of volume
1:38:00
![]() Effect of temperature change
1:40:34
Effect of temperature change
1:40:34


Classification of Elements and Periodicity in Properties
Class 11 Chemistry Video (Click To Watch)
NEET / CBSE / NCERT / ICSE

Chemical Bonding and Molecular Structure
Class 11 Chemistry Video (Click To Watch)
NEET / CBSE / NCERT / ICSE




Chemistry
Chemical Equilibrium-Video Lecture
Related Topics

Some Basic Concepts Of Chemistry

Chemical Equilibrium-Video Lecture

Classification of Elements and Periodicity in Properties

Chemical Bonding & Molecular Structure

Chemical Thermodynamics



Overview
In this video we will cover:
1.Example 1:- For the following equilibrium, Kc = 6.3 × 1014 at 1000 K , NO (g) + O3 (g) ⇌NO2 Both the forward and reverse reactions in the equilibrium are elementary bimolecular reactions. What is Kc ,for the reverse reaction?
2.For the equilibrium,2NOCl(g) ⇌ 2NO(g) + Cl2 (g)
the value of the equilibrium constant, Kc is 3.75 × 10–6 at 1069 K. Calculate the Kp for the reaction at this temperature?
3. The value of Kc for the reaction 2A ⇌ B + C is 2 × 10–3. At a given time,the composition of reaction mixture is [A] = [B] = [C] = 3 × 10–4 M. In which direction the reaction will proceed?
4.13.8g of N2O4 was placed in a 1L reaction vessel at 400K and allowed to attain equilibrium N2O4(g) ⇌ 2NO2 (g)
The total pressure at equilbrium was found to be 9.15 bar. Calculate Kc,Kp and partial pressure at equilibrium.
5.One mole of H2O and one mole of CO are taken in 10 L vessel and heated to 725 K. At equilibrium 40% of water (by mass) reacts with CO according to the equation, H2O (g) + CO (g) ⇌ H2(g) + CO2(g)
Calculate the equilibrium constant for the reaction?
6.Hydrolysis of sucrose gives, Sucrose + H2O ⇌ Glucose + Fructose Equilibrium constant Kc for the reaction
is 2 ×1013 Calculate ∆G0 at 300K.
7.Example:-Dihydrogen gas is obtained from natural gas by partial oxidation with steam as per following endothermic reaction:
CH4 (g) + H2O (g) ⇌ CO (g) + 3H2 (g)
(a) Write as expression for Kp for the above reaction.
(b) How will the values of Kp
and composition of equilibrium mixture be affected by
(i) increasing the pressure
(ii) increasing the temperature
(iii) using a catalyst ?
8. Class 11 Questions from Equilibrium class 11
9. Competitive exam questions from class 11 Equilibrium 10. IIT, JEE, CBSE PMT, questions from Equilibrium class 11
11. NCERT Solutions Class 11 Chemistry Equilibrium
All About: Equilibrium Class 11 Chemistry NCERT Chapter 6
Class 11 Chemistry seems to be a quite difficult subject for a lot of students. But, if you get a very good conceptual understanding of the subject, it can be very interesting for you. We, at neetbharath, will give our best to make Class 11 Chemistry Equilibrium NCERT Chapter 6 super-duper easy for you. Learn about Equilibrium Class 11 Chemistry in detail with experts at neetbharath.in. Concept Will Be Crystal Clear is what we promise. Learning is a lifelong process. As a student, your focus should always be on learning new facts, concepts and tricks so that you can apply them in real-life scenarios. If your conceptual understanding is clear, you will anyway perform well in your exams. So, please do not just study for the fear of exams or just to score marks, instead learn for the love of learning. We have seen many students mugging up concepts without understanding, which, in turn, makes the subject more difficult and boring for them in the future. Always remember, if your basics are strong, you will be able to understand even the most complex concepts.
We aim at making learning fun as well as engaging for you with our complete end-end learning content with Equilibrium Class 11 Chemistry Best videos, NCERT pdf download, Notes, complete syllabus of the textbook, tests, and Practice Questions. neetbharath.in (formerly called ExamFear Education) is a Free Education platform with more than 10,000 videos on Physics, Chemistry, Maths, Biology, English, Science experiments, tips & tricks, and motivational videos for Classes 6 to 12, NEET, JEE. Learn for Free!
Our team has been on a mission “To make Quality education affordable and accessible to every child across the country” since 2011. We have been one of the pioneers to start teaching through YouTube. Feel free to connect with us on social media:
We, at neetbharath, will provide you with the best quality videos on Class 11 Chemistry Equilibrium with special focus on concepts and a lot of examples, also you can download pdf. Not just that, a quick easy-to-refer and understand Equilibrium Class 11 Chemistry NCERT Chapter 6 Notes, based on the revised NCERT textbooks. You can completely rely on the study materials created by our experts and can definitely score very well in your exams. You also have the option to download pdf as well as complete all topics of the textbook. All of these mentioned above will be accompanied with:
- Class 11 Chemistry Equilibrium NCERT Chapter 6 DPPs: A set of 20 questions from each chapter for you to attempt and check your understanding. Solutions are also available and can be downloaded in pdf format. Download Equilibrium Class 11 Chemistry NCERT Chapter 6 DPPs solutions PDF for free. Practice these questions once you have watched the videos of the chapter; try to answer on your own. Once done, refer to the solutions to check if you were right or not. This would improve your problem-solving skills as well as help you revise topics you are less confident with. In fact, our research says that the majority of the students feel that their concepts are clear while attending a class or watching a lecture video. But it is during solving a problem or numerical that they truly get to know if they have understood the concept or not. Therefore, we strongly recommend you to solve the DPPs as soon as you have watched all the videos of that chapter.
- Class 11 Equilibrium Online Tests: For every chapter, you can take a test of 10 Multiple choice questions. Correct answers along with explanations will be provided after you submit the test. A set of new 10 questions will appear when you try to attempt the test for the same chapter again. This will help you to revise your concepts on Equilibrium Class 11 Chemistry. Self-evaluation is one of the best ways for you to understand how much clarity you have got on the concepts of that topic. Based on where you are getting stuck, you can give extra focus on those topics. Taking tests frequently always helps you improve your speed and accuracy.
- Equilibrium Class 11 NCERT solutions: All questions from NCERT textbook are solved in detail, along with video solutions for each question. Download Equilibrium Class 11 NCERT solutions PDF for free. Students often get stuck while solving the questions in the exercises of NCERT textbook. You can watch the NCERT solutions in the form of videos with our experts, and can also refer to pdf format on our website as well as app. If you are a student of CBSE or preparing for exams for NEET, JEE, Civil services, NDA etc. NCERT textbooks are very important for you. Please note that we have uploaded videos as per the Revised NCERT textbooks (2023-24). Therefore, you can completely rely on the content on our website/app.
- Class 11 Chemistry Sample papers: You must attempt a couple of our specially curated sample papers before appearing for your exam. This will boost your confidence and also help you practice. Once you have completed the syllabus, it is very important for you to solve Sample papers. In an exam-like environment, solve the sample paper. Once done, check the answers and understand which are your strong areas and which are the weaker ones. The sample papers are created based on the complete syllabus of a particular subject.
- Class 11 Chemistry Previous Years’ Questions: Questions that appeared in the last 5-7 years along with detailed solutions for better understanding for the entire Class 11 Chemistry syllabus. This is a very critical step towards your preparation for any examination. This gives you a fair idea of what kind of questions are asked in that particular exam. It also helps you understand the important topics from where the majority of questions are framed. We will provide you with Previous years’ questions of Class 10 and Class 12 NCERT exams, as well as NEET, JEE, both in video format as well as PDF format.
- Last but not least, We, at neetbharath, are on a mission to Promote Top Class 11 Education.
If you like our content, Spread the word!
Tips for Students- Never miss a video on Equilibrium Class 11 Chemistry
- Make your notes while watching Equilibrium Chemistry Class 11 | Chapter 7 Chemical Equilibrium One Shot | CBSE Class 11 JEE
- Make a note of all your doubts as you watch Equilibrium Chemistry Class 11 | Chapter 7 Chemical Equilibrium One Shot | CBSE Class 11 JEE, and ask them in the “Ask Question” section of neetbharath.in
- How should I prepare for my Class 11 Chemistry exam?
- Watch all our Videos, Notes, NCERT Solutions, DPPs for Class 11 Chemistry
- Download PDF of Solutions, complete syllabus of textbooks is covered in the videos
- Attend LIVE classes to ask your doubts on Class 11 Chemistry
- Can I crack NEET/JEE/Board exam using neetbharath?
Of course, YES! If you are determined to crack NEET, you can at ease do that with neetbharath.in
- Watch Class 11 Videos on Physics, Chemistry, Biology
- Ask your doubts in NEET/JEE/Board exam LIVE classes
- Refer Notes, DPPs, Online Tests, Practice questions, sample papers, DPPs for NEET
- Can I get a good score in Class 11 Chemistry board exam if I study only from neetbharath?
Yes, you can. Refer Equilibrium Class 11 Chemistry NCERT Chapter 6 videos, notes, tests, Daily practice problems, questions, sample papers. Similarly, cover all chapters of the complete syllabus of NCERT for Class 11 Chemistry
- How to make use of neetbharath the best possible way to understand Equilibrium Class 11 Chemistry NCERT Chapter 6?
- Step 1. Watch all videos, without skipping any video for Equilibrium Class 11 Chemistry
- Step 2. Ask your doubts in Ask questions section of neetbharath.in
- Step 3. Try DPPs, Revise notes, Download PDF for DPPs solutions, refer NCERT solutions for Equilibrium Class 11 Chemistry
- Step 4. Take online test, sample paper for Equilibrium Class 11 Chemistry
- Can I ask my doubts on neetbharath?
Yes. You can ask your doubts in the Ask Question section of neetbharath.in. Alternatively, you can also ask questions on our Android neetbharath app. We will try to answer your questions within 24 hours.
How to ask a doubt on NEET Bharath Tutorials : Ask Doubts With Teachers
- Are all content on neetbharath free? or some courses are paid as well?
neetbharath is a Free learning platform. neetbharath provides the best videos, notes, pdf, tests, practice questions, DPPs absolutely free of cost. Hope you have watched Free videos on Equilibrium Class 11 Chemistry. Did you like them? neetbharath
If you like the video on Equilibrium Chemistry Class 11 | Chapter 7 Chemical Equilibrium One Shot | CBSE Class 11 JEE, Do not forget to Share with your friends.
![]() Introduction
0:00
Introduction
0:00
![]() Equilibrium
0:50
Equilibrium
0:50
![]() Dynamic Equilibrium
3:39
Dynamic Equilibrium
3:39
![]() Dynamic Physical Equilibrium
9:16
Dynamic Physical Equilibrium
9:16
![]() Solid Liquid Equilibrium
10:16
Solid Liquid Equilibrium
10:16
![]() Liquid-Vapour Equilibrium
12:01
Liquid-Vapour Equilibrium
12:01
![]() Solid-Vapour Equilibrium
12:27
Solid-Vapour Equilibrium
12:27
![]() Dissolution of Solid in Liquids
14:43
Dissolution of Solid in Liquids
14:43
![]() Dissolution of Gases in Liquids
16:38
Dissolution of Gases in Liquids
16:38
![]() Characteristics of Physical Equilibrium
19:25
Characteristics of Physical Equilibrium
19:25
![]() Chemical Equilibrium
20:02
Chemical Equilibrium
20:02
![]() Chemical Equilibrium Example
25:33
Chemical Equilibrium Example
25:33
![]() Reversible reactions:Examples
26:33
Reversible reactions:Examples
26:33
![]() Depiction of Equilibrium
27:33
Depiction of Equilibrium
27:33
![]() Chemical Equilibrium:Characteristics
29:35
Chemical Equilibrium:Characteristics
29:35
![]() Law of Mass Action
31:44
Law of Mass Action
31:44
![]() Equilibrium Constant
33:03
Equilibrium Constant
33:03
![]() Law of chemical equilibrium
36:02
Law of chemical equilibrium
36:02
![]() Steps for writing equilibrium constant
39:08
Steps for writing equilibrium constant
39:08
![]() Equilibrium constant in Gaseous System
43:29
Equilibrium constant in Gaseous System
43:29
![]() Hetergeneous 7 Homogeneous Mixture
51:44
Hetergeneous 7 Homogeneous Mixture
51:44
![]() Characteristics of Equilibrium constant
52:40
Characteristics of Equilibrium constant
52:40
![]() Applications of Equilibrium constant
1:02:13
Applications of Equilibrium constant
1:02:13
![]() Predict the direction of the reaction
1:05:10
Predict the direction of the reaction
1:05:10
![]() Example on predicting the direction of the reaction
1:07:27
Example on predicting the direction of the reaction
1:07:27
![]() Example 1 on calculating equilibrium constant
1:10:31
Example 1 on calculating equilibrium constant
1:10:31
![]() Example 2 on calculating equilibrium constant
1:17:10
Example 2 on calculating equilibrium constant
1:17:10
![]() K,Q And G relationship
1:21:43
K,Q And G relationship
1:21:43
![]() Example : K,Q And G relationship
1:26:38
Example : K,Q And G relationship
1:26:38
![]() Factors affecting equilibrium
1:27:32
Factors affecting equilibrium
1:27:32
![]() Le Chatelier's Principle
1:30:07
Le Chatelier's Principle
1:30:07
![]() Effect of concentration change
1:31:43
Effect of concentration change
1:31:43
![]() Effect of change of pressure
1:34:24
Effect of change of pressure
1:34:24
![]() Effect of change of volume
1:38:00
Effect of change of volume
1:38:00
![]() Effect of temperature change
1:40:34
Effect of temperature change
1:40:34

 9868233590
9868233590